Synthesis and Electrochemical Properties of a New Molybdenum (VI) Complex with Schiff Base Ligand

The reaction of 2-pyridylamino-N,N-bis(2-methylene-4,6-bitert-butylphenol) (H2L’) and MoCl5 gives a molybdenum (VI) complex [MoL’(O)2] 1, which has been characterized by single-crystal X-ray diffraction, IR and NMR analysis. Electrochemical studies show that 1 can electrocatalyze hydrogen evolution, both from acetic acid with a turnover frequency (TOF) of 36.4 moles of hydrogen per mole of pre-catalyst per hour at an overpotential of 441.6 mV (in DMF), and from buffer (pH 7.0) with a TOF of 373.1 moles of hydrogen per mole of catalyst per hour at an overpotential of 787.6 mV. Hydrogen is one of the most ideal energy in the future, because of its potential to reduce the current dependence on fossil fuels. Effective proton reduction to form H2 has been a subject of intense study and significant effort has been made to design metal complexes for proton reduction. In Nature, hydrogenase enzymes can efficiently catalyze both the production and oxidation of hydrogen using earth-abundant metals (nickel and iron). However, enzymes are difficult to adapt for commercial applications and their stability is often limited outside of their native environment. Electrolysis of water is the simplest way to produce hydrogen. To increase the reaction rate and lower the overpotential, it is necessary to use an efficient hydrogen evolution reaction (HER) electrocatalyst. Many research groups, including ours, have focused on the development of molecular catalysts employing the more abundant metals, and several complexes that contain nickel, cobalt, copper and molybdenum have been developed as electrocatalysts for the reduction of water to form H2. A report from Chang and co-workers described a highly active molecular molybdenum(IV) electrocatalyst, [(Py5Me2)MoIVO]2+ (Py5Me2=2,6- bis(1,1-bis(2-pyridyl)ethyl)pyridine, a neutral pentadentate ligand) that reduces water to H2 at neutral pH in aqueous buffer, reporting a maximum of 1,600 moles of H2 per mole of catalyst per hour at an overpotential of 642 mV. To investigate the roles of the oxidation/reduction site in molybdenum complexes, determining of their redox potentials and characterization of their electronic structures, we focus our work in the design and catalytic properties of molybdenum (VI) complexes.
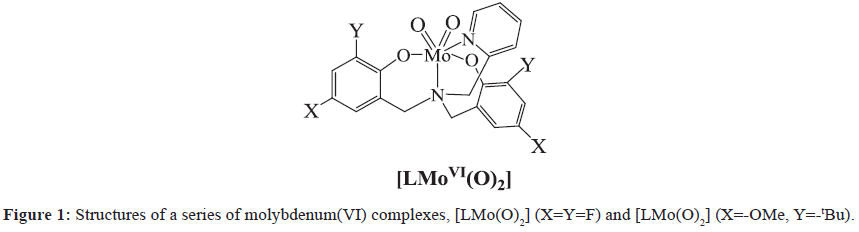
We have previously shown that the molybdenum (VI) complexes supported by 2-pyridylamino-N,N-bis-phenol (Figure 1) also can be molecular electrocatalysts for hydrogen evolution. It has been shown that the donor type and electronic properties of the ligands play vital roles in determining the structure and reactivity of the corresponding metal complexes. With this mind, we chose 2-pyridylamino-N,Nbis( 2-methylene-4,6-bitert-butylphenol) (H2L’), a potential deprotonated ligand to react with MoCl5 to assembly the corresponding molybdenum compound and study its electrocatalytic function. Here we present the synthesis, structure, characterization and properties of a new molybdenum (VI) electrocatalyst, [MoL’(O2)] 1, as well as the effect of tetradentate ligand modifications on the catalytic properties of molybdenum (VI) complexes.
For more info kindly visit: https://www.imedpub.com/der-chemica-sinica/
With Regards,
Joseph Kent
Journal Manager
Journal of Der Chemica Sinica