Synthesis and Antimicrobial Activity of 2-Benzylidene-1,3 Indandiones: A Structure-Reactivity Study

Substituted 2-benzylidene-1,3-indandiones have been prepared and characterized by UV, IR, 1H and 13C NMR spectral analysis. The antimicrobial activities and structure reactivity correlation of the compounds have been studied. Different methods have been used for the synthesis of 1,3-indandione derivatives with substitution at position 2. Previous studies reported phenylation of 1,3-indandione with diaryliodonium salts and α-alkenylation of β-dicarbonyl compounds with alkenyltriarylbismuthonium salts. The Friedel-Crafts methods were also reported for the derivatization of 1,3-indandione at position 2. In addition to these conventional methods, the electrochemical synthesis has also been used for preparation of indandione derivatives with catechol or 2,3-dimethylhydroquinone ring on their position 2. Studies of substituent effects on zone of inhibition against the growth of microorganisms in various substituted N-(1-piperidino benzyl) nicotinamide and substituted N-(1-piperidinobenzyl) acetamide and substituted N-(1- morpholinobenzyl) acetamide have been reported.
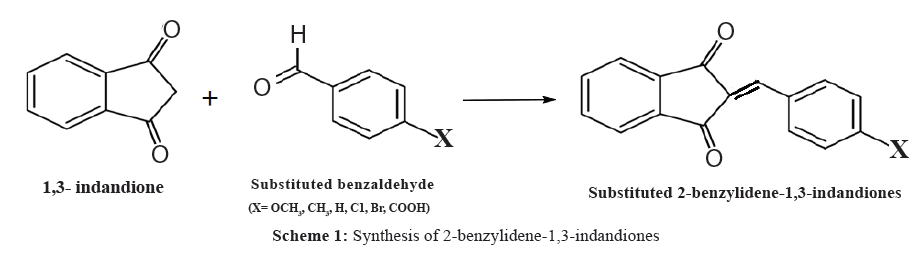
The literature reveals that there is a little work done on the antimicrobial study of activated olefinic compounds. As a part of our interest in the structure-reactivity study, we have synthesized 2-benzylidene-1,3-indandiones and studied the antimicrobial activity to find out the substituent effect on 2-benzylidene-1,3-indandione. All chemicals used were purchased from Sigma Aldrich. Purity of the compounds was checked by TLC on silica gel G plates. UV-Visible spectra were recorded on a CARY VARION (V 550), CHCl3 was used as a solvent. Infrared spectra were obtained on a PARAGON 500 spectrometer on KBr pellets. 1H and 13C spectra were obtained on a BRUKER AMX 400 MHz spectrometer. Chemical shift of 1H were measured with the peak of CDCl3 at δ 7.29 as the internal reference, while those of 13C were recorded with the central peak of CDCl3 at δ 77.03 as the internal reference.
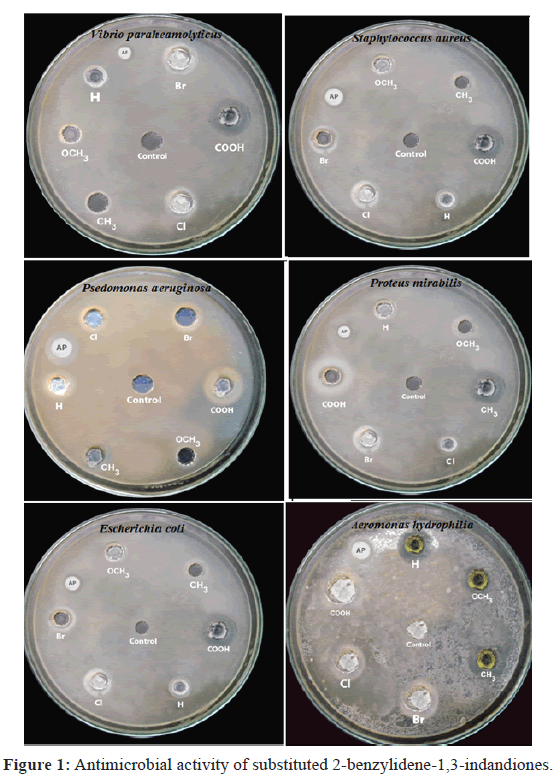
To summarize, substituted 2-benzylidene-1,3-indandiones have been synthesized and evaluated for their antibacterial activities. This reaction protocol offers a simple, easier work-up procedure and good yields. The compounds have been characterized by their spectral data. The antimicrobial activities of all synthesized compounds have been studied. The inhibition zone radii of these compounds have been correlated with Hammett substituent constants, F and R parameters. From the results of statistical analysis, the effects of substituent on the antimicrobial activity of compounds have been studied.
Warm Regards,
Joseph Kent
Journal Manager
Journal of Der Chemica Sinica