Inhibition of Corrosion of Zinc in H2SO4 Medium by the Schiff Base, 4-Hydroxy Phenyl Methylidene-2-(1-Phenyl Ethylidene) Hydrazine Carbothioamide (4-HPMHC)

Inhibitive effects of Schiff’s base, 4-hydroxy phenyl methylidene-2-(1-phenyl ethylidene) hydrazine carbothioamide (4-HPMHC) on the corrosion of zinc in H2SO4 at temperatures 303 K, 313 K and 323 K were studied using weight loss method. Inhibition efficiency of 4-HPMHC was found to depend on concentrations of inhibitor and the acid, temperature of the process and period of contact. The study revealed that 4-HPMHC inhibits the corrosion of zinc with maximum inhibition efficiency of 86.87% at 0.0005 M of the inhibitor. The kinetics of uninhibited and inhibited corrosion reaction was found to follow first order. Activation energy (Ea) value (18.22 J mol-1) of the inhibited corrosion system was higher than that of the uninhibited system with value of 4.919 J mol-1 at 0.0005 M of inhibitor and in 0.05 M of the H2SO4 acid. The calculated thermodynamic parameters viz; enthalpy, entropy and free energy of the inhibited reaction suggest that the process is spontaneous and exothermic. An adsorption characteristic of the studied inhibitor was best described by Langmuir adsorption isotherm.
The Schiff’s base (4-HPMHC) was synthesized by the method reported in Mandell et al. and Hemandez-Molina et al. Zinc specimens used for the study were of dimensions 4 cm × 4 cm × 0.10 cm. The acid solutions (0.01 M to 2.0 M H2SO4) were prepared from analytical grade and were used for the weight loss measurement. The zinc coupon was washed in ethanol, dried in acetone and preserved in a desiccator. Various concentrations (0.0001 M to 0.0005 M) of the inhibitor, 4-HPMHC was also prepared.
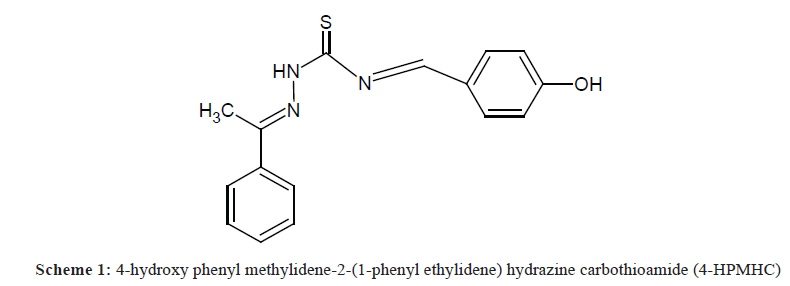
A very important process in the area of prevention and control of corrosion is the use of organic inhibitors. The introduction of organic inhibitors is in consonance with the focus of chemistry in the 21st century, which centers mostly on green chemistry. Organic inhibitors are found to be safer, eco-friendly and biocompatible when compared with their inorganic counterparts. An important factor to be put into consideration in the mechanism of organic inhibitors when employed as corrosion inhibitors is the exclusive interaction between certain functionalities in the inhibitors with the active sites in the metallic surface. A corrosion inhibitor is generally referred to as a chemical substance that when applied in small quantities to a corrosive medium reduces the rate of corrosion of a metal or a metal alloy. A search for a suitable inhibitor for the corrosion of metals involves a choice between those synthesized from cheap raw materials and those of organic origin containing hetero-atoms (N, O, S or P) in aromatic or long chain carbon compounds. Schiff’s bases possess hetero-atoms such as nitrogen, oxygen and sulphur, which play major roles in this interaction by donating free electron pairs to the active sites of the metal surface. In addition, the presence of double bonds in Schiff’s bases makes them efficient inhibitors due the availability of π-electrons for interaction with metal surface. Compounds containing the aforementioned hetero-atoms in addition to the π-electrons have been found to display strong inhibition characteristics. The present study seeks to use a synthesized Schiff’s base, 4-hydroxy phenyl methylidene-2-(1-phenyl ethylidene) hydrazine carbothioamide (4-HPMHC) to inhibit the corrosion of zinc in H2SO4 medium. The structure of the Schiff base used in the corrosion study is given in Scheme 1. From the structure, it is indicative that the 4-HPMHC molecule contains hetero-atoms and may be a good inhibitor for the corrosion of zinc in H2SO4.
From the results of the study carried out, the following conclusions can be drawn:
• 4-HPMHC is a good inhibitor for the corrosion of zinc in 0.01 M to 0.05 M concentration of H2SO4.
• Inhibition efficiency of 4-HPMHC is dependent on concentration, temperature and period of exposure.
• Inhibited corrosion reaction of the Zn metal in the presence of 4-HPMHC follows first order kinetics.
• Thermodynamic parameters calculated show that adsorption of 4-HPMHC on zinc surface is exothermic, spontaneous and is accompanied by decreasing disorderliness.
• The adsorption of the 4-HPMHC on zinc surface proceeds via chemical adsorption mechanism and the adsorption process could best be described by Langmuir adsorption isotherm.
With Regards,
Joseph Kent
Journal Manager
Journal of Der Chemica Sinica