Effect of a Polyherbal Dietary Supplement on Semen Characteristics: A Randomized, Double-Blind Placebo-Controlled Clinical Trial

Ejaculate volume, an important semen characteristic, plays a significant role in male fertility. There is a growing need to find supplements to increase ejaculate volume. The primary objective of the study was to evaluate the efficacy and safety of a dietary supplement (SemenaxTM) on semen characteristics of hypospermic and normospermic men. A double-blind, randomized placebo-controlled exploratory clinical trial. Six clinical sites 3 at Nasik, 2 at Pune and 1 at Mumbai, Maharashtra, India. Males aged 30 Years-60 years (n=78) diagnosed with hypospermia/normospermia with low ejaculate volume; normozoospermia; mild to moderate oligozoospermia; impairment of sperm motility and morphology and erectile dysfunction. The participants were randomized in a ratio of 1:1 to receive the suppleent (3200 mg in two divided doses) or placebo for two months. Main outcomes were mean change in ejaculate volume, sperm characteristics, sexual function, and orgasm quality from baseline to end of the study compared to placebo. In addition the safety of the supplement was evaluated by questionnaires, vitals and laboratory parameters. The supplement significantly increased the ejaculate volume from the baseline values compared to the placebo (19.67% vs. 8%). The number of responders in the supplement group was significantly more than in the placebo group (50% vs. 16%). Orgasm quality was improved in 65.63% of participants compared to 41.9% in placebo. No major safety concerns were reported in both groups. Overall, the dietary supplement effectively improved the ejaculate volume to significant levels without any significant adverse effects.
Ejaculate comprises the concentrated suspension of spermatozoa with L-carnitine and neutral alpha-glucosidase, stored in the paired epididymides (10% of the volume), diluted by prostatic secretions (10% of the final semen volume) containing acid phosphatase, citric acid, inositol, calcium, zinc and magnesium in the urethra and the fructose, ascorbic acid, and prostaglandins-containing seminal vesicle fluid (75%-80% of the final seminal fluid). A mucus-rich secrete from Cowpers' or bulbourethral glands forms a minor part of the ejaculate and is the first to appear during ejaculation. Adequate ejaculate volume is necessary to transport sperms in the female reproductive tract for fertilization. Also, the spermatozoa ejaculated in prostatic fluid preserve better motility and vitality than when expelled in seminal vesicular fluid. However, during the etiological screening of male infertility, the role of ejaculate volume as a causative factor is often neglected.
The present study was a randomized, double-blind, placebo-controlled, parallel-group, multi-center study to assess the efficacy and safety of the dietary supplement capsules on semen characteristics of hypospermic and normospermic men (n=78) after two months of oral administration. The main objectives of the study were to evaluate the efficacy of the dietary supplement on 1) The ejaculate volume of hypospermic and normospermic men, 2) Sperm characteristics including count, morphology, and motility, 3) Sexual function using the International Index of Erectile Function (IIEF), and 4) Orgasm quality. The secondary objective of the study was to assess the safety and tolerability of the dietary supplement in comparison to the placebo. For subgroup analysis the two groups were further stratified as hypospermic and normospermic. The details of the study flow and the visit schedule are provided in Figure 1 (Figure 1).
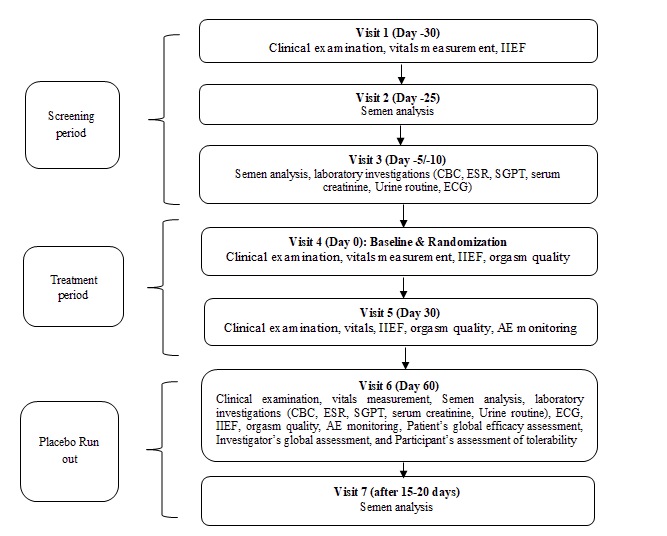
Participants were screened for the eligibility criteria defined for the study over the three screening visits. The eligible participants were then randomized to the study groups and provided with the IP. Subsequently, monthly follow-up study visits were conducted on day 30 and day 60 followed by a placebo run-out period of 15 days-20 days. The assessments done on each visit are described in Figure 1. At the end of the placebo run-out period again the semen analysis was performed. Treatment compliance was ensured by recording dispensed and returned medication at each visit. In addition, consumption of any concomitant medication was documented in the case report form.
As this was the first study of this supplement, therefore, based on previous similar studies a minimum sample size of 60 evaluable participants, with 30 in each group, was considered appropriate to detect a statistically significant difference between the supplement and placebo. The efficacy analyses were done on the Per-Protocol (PP) population comprising participants reporting for all protocol stipulated study visits and did not have any major protocol deviations related to the primary efficacy outcome while the safety analyses were performed on the Intention-To-Treat (ITT) population
Descriptive statistics are presented as absolute counts, percentages, mean, and Standard Deviation (SD). Continuous baseline characteristics, efficacy, and safety parameters were compared using Analysis of Variance (ANOVA). Subgroup analysis was performed for two sub-groups: Normospermic and hypospermic. Pearson's Chi-square test was applied to analyze the categorical parameters such as grade of orgasms, number of responders, and participant's global efficacy and tolerability assessment, as well as investigator's global assessment. All statistical tests were performed at a 5% level of significance.
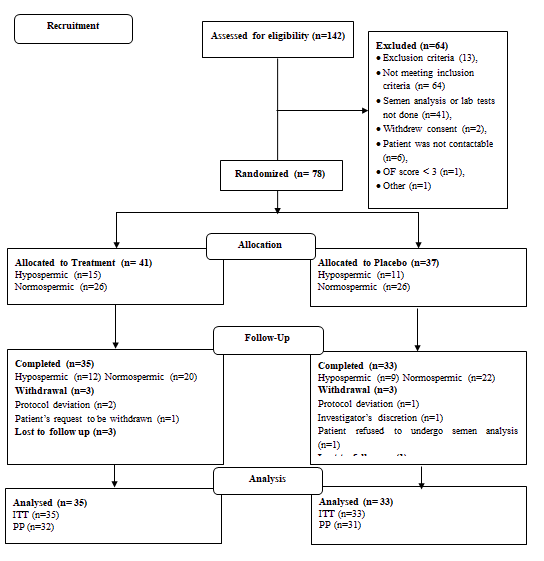
A total of 142 study participants were screened for the study, of which 64 were screening failures. The remaining 78 study participants were randomized to receive either the supplement (n=41) or placebo (n=37). A comparable number of hypospermic and normospermic men were included in both groups for subgroup analysis. The detailed participant disposition is provided in Figure 2 (Figure 2).
The current study was an exploratory, randomized, double-blind, and placebo-controlled clinical investigation to assess the safety and efficacy of the dietary supplement in men desiring improvement in semen quality. The study results showed that the supplement could improve the ejaculate volume significantly after two months of administration compared to the placebo. On subgroup analysis, it was revealed that this effect was more pronounced in the healthy normospermic population. On the other hand, in the hypospermic population, it could not reach the significance level.
The supplement group showed more responders than the placebo group, with the difference between the two groups being statistically significant. Again the efficacy of the supplement was more in the normospermic subgroup. For sperm characteristics, the hypospermic group performed statistically better in improving the morphology, while the normospermic group showed improvement in non-progressive motility of the sperms. Overall, the polyherbal dietary supplement has demonstrated efficacy in improving the ejaculate volume and orgasm quality to statistically significant levels in the study participants. It has demonstrated a good acceptability and safety profile. The study opens new avenues for men desiring to improve their semen quantity and sperm quality with better orgasm.
With Regards,
Joseph Kent
Journal Manager
Journal of Clinical Nutrition & Dietetics.