Design, Synthesis and Pharmacological Evaluation of New Series of 2-Pyrazoline Containing s-Triazine and their Derivatives

In the present investigation, a series of some novel 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl-1H-pyrazol-3-yl) Substituted phenyl)-1,3,5-triazin-2-amine 7(a-h) have been synthesized by the treatment of 1-(4-(4,6-diethoxy-1,3,5- triazin-2-ylamino)phenyl)-3-substituted phenyl prop-2-en-1-one (CHAlcone) (6a-6h) with hydrazine hydrate in DMF. The structure of newly synthesized compounds was confirmed by the IR, 1H NMR and Mass spectral analysis. All the synthesized compounds were evaluated for anti-fungal and anti-bacterial activity. Most of the compound showed potent activity. Heterocyclic compounds play important roles in the drug discovery process, substituted heterocyclic compounds offer a high degree of structural diversity and have proven to be broadly useful as therapeutic agents. Of these heterocycles 1,3,5-triazine core have been reported to possess a wide range of biological activities. These include antiviral and anticancer anti-tuberculosis, antibacterial, antifungal, antimalarial, antiviral, herbicidal, anesthetic and anti-inflammatory activities. Moreover, 1,3,5-triazine are useful intermediates in the construction of several other heterocycles. Pyrazoline are prominent nitrogen containing five membered heterocyclic bioorganic molecules, which occupy unique position in medicinal chemistry, due to the broad range of pharmacological activities. They are known to possess antibacterial, anticancer, antioxidant and anti-inflammatory activities. In view of these inspections and in persistence of the research work on 1,3,5-triazine and 2-Pyrazoline and in continuation of our research program. It was thought of interest to merge both 1,3,5-triazine and 2-Pyrazoline moieties which may enhance the drug activity and viewing them for antimicrobial activities. In the present work, 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl-1H-pyrazol-3-yl) Substituted phenyl)-1,3,5-triazin-2-amine (7a-7h) have been synthesized by the treatment of 1-(4-(4,6-diethoxy-1,3,5 triazin-2-ylamino)phenyl)-3-substituted phenylprop-2- en-1-one (CHAlcone) (6a-6h) with hydrazine hydrate in DMF.
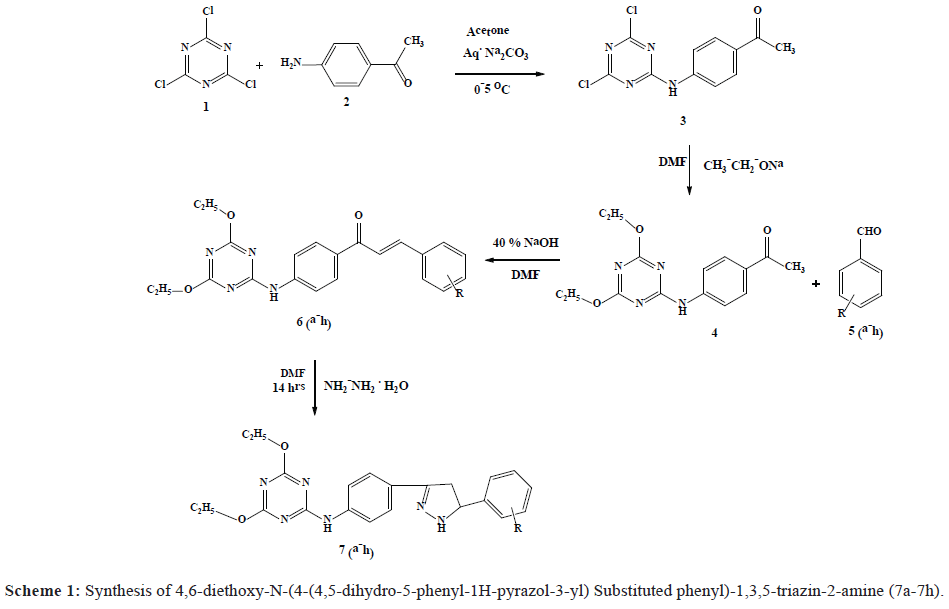
(Scheme 1). The structures of all synthesized compounds were assigned on the basis of IR, Mass, 1H NMR spectral data and elemental analysis. Further these compounds were subjected for antifungal and antibacterial activity.
Melting points were determined in open capillaries and are uncorrected.IR spectra were recorded using Perkin Elmer spectrometer. 1H NMR spectra were recorded on Brucker Advance II 400 spectrometer in DMSO-d6 by using TMS as internal standard. Thin layer chromatography was performed with E. Merk precoated TLC plates, silica gel 60F254 with thickness of 0.25 mm and spots were visualized by irradiation with ultraviolet light (254 nm). The synthesis of compounds substituted 1-(4-(4,6-diethoxy-1,3,5-triazin-2-yl-amino)phenyl)-3-phenylprop-2-en-1- one (CHAlcone) (6a-6h) was accomplished by reacting 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)ethanone (4) with substituted benzaldehyde (5a-5h) in DMF. The CHAlcones (6a-6h) underwent ring closure via condensation with hydrazine hydrate to give substituted 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl-1H-pyrazol-3-yl)phenyl)-1,3,5- triazin-2-amine (7a-7h). The synthetic pathway followed for the synthesis of the title compounds is described in Scheme 1.
With Regards,
Joseph Kent
Journal Manager
Journal of Der Chemica Sinica